SI Joint Fusion: Treatment for Sacroiliac Joint Pain
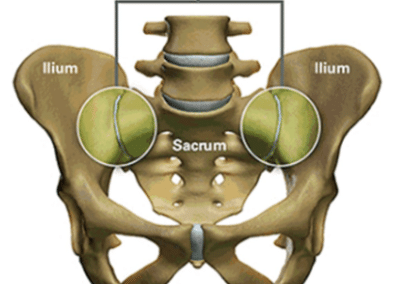
The sacroiliac joint (SI joint), located in the pelvis and linking the pelvis to the sacrum (the lowest part of the spine above the tailbone), can be a significant cause of lower back pain.
Like any other joint in the body, the SI joint can be injured and/or undergo degeneration. When this happens, people can feel pain in their buttock and sometimes in the lower back, hips and legs. This is especially true while lifting, running, walking or even lying on the involved side.
It’s common for pain from the SI joint to feel like disc or lower back pain, or sometimes hip or groin pain. For this reason, SI joint disorders should always be considered in lower back, hip, and pelvic pain diagnosis.
Who Benefits from SI Joint Fusion
You may be a candidate for SI joint fusion if you have:
- Lower back pain
- Sensation of low extremity: pain, numbness, tingling, weakness
- Pelvis/buttock pain
- Hip/groin pain
- Feeling of leg instability (buckling, giving way)
- Disturbed sleep patterns due to pain
- Disturbed sitting patterns (unable to sit for long periods, sitting on one side)
- Pain going from sitting to standing



What to Expect
The most relied upon method to accurately determine whether the SI joint is the cause of your lower back pain symptoms is to inject the SI joint with a local anesthetic. This diagnostic injection at iSpine Clinics is performed under X-ray guidance to verify accurate placement of the needle in the SI joint. If your symptoms decrease it can be concluded that the SI joint is either the source of or a major contributor to your lower back, hip, or pelvic pain. If the level of pain does not change after SI joint injection, it is less likely that the SI joint is the cause of your pain.
SI Joint Fusion with iFuse TORQ® Implant System
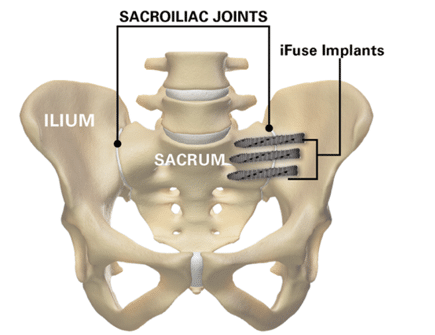
If diagnosis supports SI fusion, iFuse TORQ® is designed to stabilize and fuse the SI joint. The iFuse procedure involves inserting typically three threaded titanium implants across the SI joint to maximize stability, reduce pain, and improve function. The procedure is done through a small one-inch incision and takes about an hour. The 3D-printed iFuse TORQ implant was designed for osseointegration, which is the structural and functional connection between implant and bone. This allows your painful joint to be stabilized through binding of bone all along the implant.
Preparation: You’ll have injection and imaging studies beforehand.
Anesthesia: Usually performed under local anesthesia with light sedation.
Technique:
- A small incision (about 1 inch) is made for implant insertion.
- Using fluoroscopic (X-ray) guidance, the physician inserts three stabilizers through the ilium and into the sacrum.
- The implants are designed so that bone will grow into and around them, stabilizing the joint long-term.
Duration: The procedure typically takes about 1 hour. You will be monitored afterward and usually discharged the same day.
iFuse TORQ® Implant System is one of the latest innovative solutions from SI-BONE, the creator of the minimally-invasive SI joint fusion device—the triangular titanium iFuse Implant. More than 100 peer-reviewed publications demonstrate the safety, durable effectiveness, and biomechanical and economic benefits of the iFuse implant (www.si-bone.com/results).
After the Procedure
- Incision Care: A small bandage covers the incision; stitches or staples may be used.
- Immediate Sensations: Mild soreness or swelling at the incision site and some low back or buttock discomfort are common for a few days.
- Mobility: You’ll typically use crutches or a walker for short distances right after surgery to limit pressure on the joint.
Recovery and Aftercare
- Weight Bearing:
- Partial weight bearing is usually recommended for the first 2–3 weeks.
- Full weight bearing is gradually increased as advised by your surgeon.
- Physical Therapy: A rehabilitation plan often begins within a few weeks to improve strength, stability, and flexibility.
- Pain Management: Over-the-counter or prescribed medications can help manage mild post-surgical pain.
- Healing Time: Initial recovery takes about 6–12 weeks, with full fusion and long-term pain relief continuing to improve for several months.
- Follow-Up Visits: Your doctor will monitor healing with periodic exams and imaging.
Risks and Considerations
As with any minimally invasive surgical procedures, there are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit www.si-bone.com/risks.
- Infection or bleeding
- Nerve irritation
- Implant movement (rare)
- Continued or recurrent pain if other pain sources exist
Explore whether iFuse is right for you
The first step to finding out if you’re a candidate for the iFuse procedure is making an appointment at iSpine Clinics to review your case and discuss your options.
To make an appointment, call 763-201-8191.
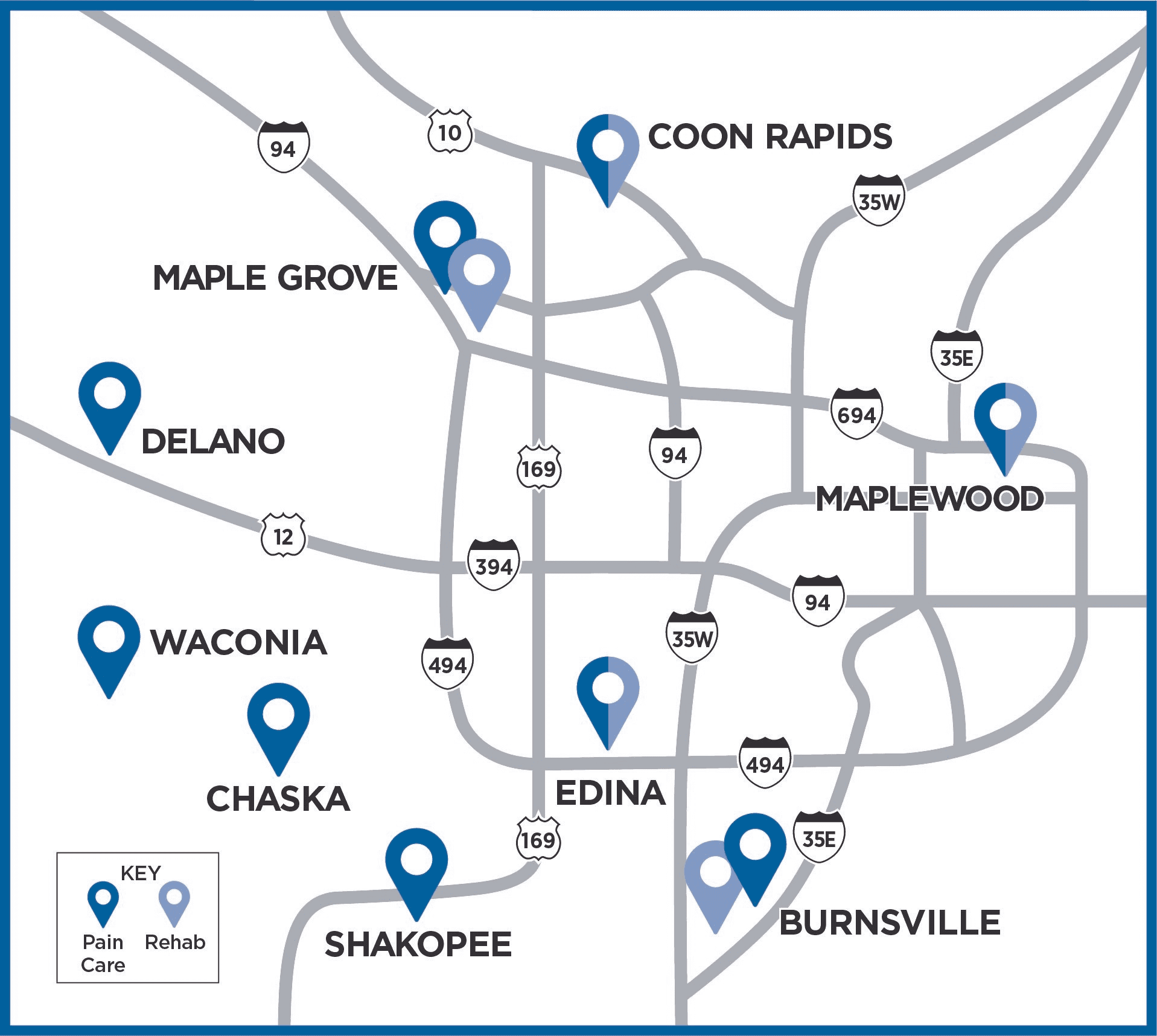
Explore the Twin Cities Metro Clinics where we evaluate patients for SI Joint Pain
*iSpine Clinic locations where iFuse procedures are conducted
References
- Bernard TN, et al. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;217:266–80.
- Schwarzer AC, et al. The Sacroiliac Joint in Chronic Low Back Pain. Spine. 1995;20:31–7.
- Maigne JY, et al. Results of Sacroiliac Joint Double Block and Value of Sacroiliac Pain Provocation Tests in 54 Patients with Low Back Pain. Spine. 1996;21:1889–92.
- Sembrano JN, et al. How Often is Low Back Pain Not Coming From The Back? Spine. 2009;34:E27–32.
- DePalma MJ, et al. Etiology of Chronic Low Back Pain Patients Having Undergone Lumbar Fusion. Pain Med. 2011;12:732-9.
- SI-BONE 300857-R.
- Polly DW, et al., and the INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016;10:Article 28. DOI: 10.14444/3028
- Dengler J, et al. Randomized Trial of Sacroiliac Joint Fusion vs. Conservative Management for Chronic Low Back Pain Attributed to the Sacroiliac Joint. J Bone Joint Surg Am. 2019;101(5):400-11. DOI: 10.2106/JBJS.18.00022.
- Duhon B, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T, on behalf of the SIFI Study Group. Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: 2-Year Follow-Up from a Prospective Multicenter Trial. Int J Spine Surg. 2016;10:Article 13. DOI: 10.14444/3013
- Dengler J, et al. on behalf of the INSITE, iMIA and SIFI study groups. Predictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint – a Pooled Analysis. Spine. 2017;42(21):1664-73. [Epub 2017 Mar 27]. DOI: 10.1097/BRS.0000000000002169
- Whang PG, et al. Long-Term Prospective Clinical and Radiographic Outcomes After Minimally Invasive Lateral Transiliac Sacroiliac Joint Fusion Using Triangular Titanium Implants. Med Devices (Auckl). 2019;12:411-422. DOI: 10.2147/MDER.S219862
- Patel V, et al. Prospective Trial of Sacroiliac Joint Fusion Using 3D-Printed Triangular Titanium Implants: 24-Month Follow-Up. Med Devices (Auckl). 2021;14:211-216. DOI: 10.2147/MDER.S314828